mo number of protons|Protons, Neutrons, Electrons for Molybdenum (Mo, Mo3+) : Pilipinas Mass Number Relative Atomic Mass Isotopic Composition; 83 Mo: 83: 82.94874(54)# 84 Mo: 84: 83.94009(43)# 85 Mo: 85: 84.93655(30)# 86 Mo: 86: 85.93070(47) 87 Mo: 87: .
系统架构. 问题描述. 在公众号token验证,通过此域名一直请求不到服务器,报token check fail,但是通过浏览器、终端访问此url均可以访问成功,. 问题定位. 询问运维是否对SLB加了白名单,运维答复没加白名单限制San Fernando (La Union) Vai alla navigazione Vai alla ricerca San Fernando città componente; San Fernando – Veduta . San Fernando è una città componente delle Filippine, capoluogo della Provincia di La Union e della Regione di Ilocos. San Fernando è formata da 59 baranggay: Abut; Apaleng; Bacsil;
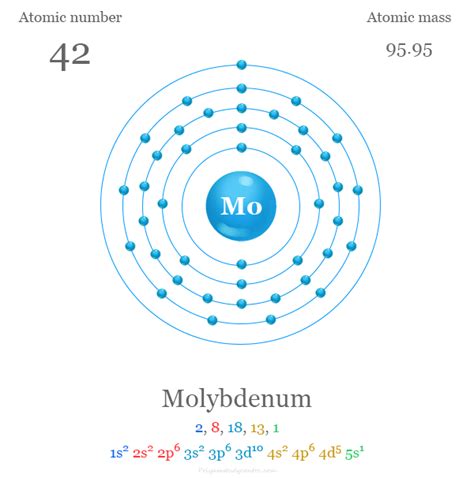
mo number of protons,Element Molybdenum (Mo), Group 6, Atomic Number 42, d-block, Mass 95.95. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Iodine has 53 protons, 74 neutrons and 53 electrons: 54: Xenon has 54 protons, 77 neutrons and 54 electrons: 55: Cesium has 55 protons, 78 neutrons and 55 electrons: 56: Barium has 56 protons, 81 .Symbol: Mo. Atomic Number: 42. Atomic Mass: 95.94 amu. Melting Point: 2617.0 °C (2890.15 K, 4742.6 °F) Boiling Point: 4612.0 °C (4885.15 K, 8333.6 °F) Number of Protons/Electrons: 42. Number of Neutrons: 54. .
Molybdenum is a chemical element with atomic number 42 which means there are 42 protons in its nucleus. Total number of protons in the nucleus is called the .
Molybdenum is the 42nd element in the periodic table and has a symbol of Mo and atomic number of 42. It has an atomic weight of 95.95 and a mass number of 98. Molybdenum .Mass Number Relative Atomic Mass Isotopic Composition; 83 Mo: 83: 82.94874(54)# 84 Mo: 84: 83.94009(43)# 85 Mo: 85: 84.93655(30)# 86 Mo: 86: 85.93070(47) 87 Mo: 87: .
The easiest way to find the number of protons, neutrons, and electrons for an element is to look at the element’s atomic number .Number of protons: 42 p + Number of neutrons: 54 n 0: Number of electrons: 42 e-Molybdenum is a chemical element with atomic number 42 which means there are 42 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The .Protons and Neutrons in Chlorine. Chlorine is a chemical element with atomic number 17 which means there are 17 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the .
Protons and Neutrons in Barium. Barium is a chemical element with atomic number 56 which means there are 56 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and .
Number of Protons: 42: Number of Neutrons: 54: Number of Electrons: 42: Melting Point: 2617.0° C: Boiling Point: 4612.0° C: Density: 10.22 grams per cubic centimeter: Normal Phase: . [Mo(CO) 6] Molybdenum hexafluoride (MoF 6) Molybdenum oxide (MoO 3) Molybdenum phosphide (MoP 2) Molybdenum trioxide (MoO 3) Number of Neutrons = Mass Number - Number of Protons = 1 - 1 = 0. For zinc, the atomic weight is 65.39, so the mass number is closest to 65. Number of Neutrons = 65 - 30 = 35. Cite this Article. Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element.Atomic Number of Molybdenum. Atomic Number of Molybdenum is 42. Chemical symbol for Molybdenum is Mo. Number of protons in Molybdenum is 42. Atomic weight of Molybdenum is 95.95 u or g/mol. Melting point of Molybdenum is 2617 °C and its the boiling point is 5560 °C.mo number of protons Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one (+1) ( + 1) and a mass of 1 atomic mass unit (amu) ( amu), which is about 1.67 ×10−27 1.67 × 10 − 27 kilograms. Together with neutrons, they make up virtually all of the mass of an atom. Boron is a chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structure.The chemical symbol for Boron is B. Significant concentrations of boron occur on the Earth in compounds known as the borate minerals. There are over 100 different borate minerals, but the most common are: borax, .
Mo – 3e – → Mo 3+. The electron configuration of molybdenum ion (Mo 3+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 3. The electron configuration of a molybdenum ion shows that the molybdenum ion (Mo 3+) has four shells and the last shell has eleven electrons. Molybdenum exhibits +4, +6 oxidation states.

Molybdenum (Mo) Molybdenum is the 42nd element in the periodic table and has a symbol of Mo and atomic number of 42. It has an atomic weight of 95.95 and a mass number of 98. Molybdenum has forty-two protons and fifty-six neutrons in its nucleus, and forty-two electrons in five shells. It is located in group six, period five and block d of the .The number of protons in an isotope atom does not change but the number of neutrons does. The zinc atom has a total of thirty isotopes. Isotope: Mass number (A) Atomic number (Z) Neutron number = A – Z: . Molybdenum is a classified transition metal and its symbol is ‘Mo’. Molybdenum is the 42nd element of the periodic table so its .

The number of protons in an isotope atom does not change but the number of neutrons does. The iodine atom has a total of thirty-seven isotopes. Isotope: Mass number (A) Atomic number (Z) Neutron .
Molybdenum (Mo) is a silvery-white metal that has the atomic number 42 in the periodic table. It is a Transition metal and located in Group 6 of the periodic table. It has the symbol Mb. . Protons. 42. .First, to find the number of protons, we need to realize that the neutral atom had 53 electrons because it is the additional one electron that makes it a 1- anion. Now, because the atom has 53 electrons, it must also have .
An atom’s mass number close mass number The number of protons and neutrons found in the nucleus of an atom. is the total number of protons and neutrons.
Protons, Neutrons, Electrons for Molybdenum (Mo, Mo3+)An atom’s mass number close mass number The number of protons and neutrons found in the nucleus of an atom. is the total number of protons and neutrons.mo number of protons Protons, Neutrons, Electrons for Molybdenum (Mo, Mo3+) Boron is a chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structure.The chemical symbol for Boron is B. Significant concentrations of boron occur on the Earth in compounds known as the borate minerals. There are over 100 different borate minerals, but the most common are: borax, .Mo Molybdenum Element 42 Mass Number: 96 Atomic weight: 95.95 g/mol Atomic number (Z): 42 Electrons: 42 Protons: 42 Neutrons: 54 Period: 5 Group: 6 Block: d atomic number- 42. chemical number- 96. number of protons-18. atomic mass-96. number of eletrons-42. number of valence eletrons- 1, 2, 13, or 18. (sorry i forgot) nummber of shells in n=3 shell-18. number of neutrons-54. Explanation: well i just know the periodic table by heart and thats how i got the answer XD Mass number of Mo is 96 it is the sum of number of protons and neutrons. For a neutral atom, the number of electrons equal to the number of protons. Thus number of protons is 42.Number of neutrons can be found by substracting proton number from mass number. number of neutrons = 96 -42 =54. The electronic configuration of Mo is .
mo number of protons|Protons, Neutrons, Electrons for Molybdenum (Mo, Mo3+)
PH0 · Protons, Neutrons, Electrons for Molybdenum (Mo, Mo3+)
PH1 · Protons Neutrons & Electrons of All Elements (List
PH2 · Molybdenum (Mo)
PH3 · Molybdenum (Mo)
PH4 · Molybdenum
PH5 · How to Find the Number of Protons, Neutrons, and
PH6 · Chemical Elements.com